Research
Peculiarities in gametes
Even if organisms reach the end of their lives at the individual level, they can survive semi-permanently at the species level by refreshing the genetic and epigenetic information in their germ cells and passing it on to the next generation accurately. In order to ensure the immortality of gametes, many unique biological phenomena are observed in germ cells. First, a specialized cell division called meiosis halves the number of chromosomes. After that, differentiation into sperms and eggs, which are specialized cells for fertilization, occurs, and genetic information is passed on to the next generation through fertilization. Our team combines mouse and nematode genetics, cytology, biochemistry, etc., and focuses on the unique biological phenomena seen only in germ cells in order to study the molecular basis behind the development and maintenace of these cells.
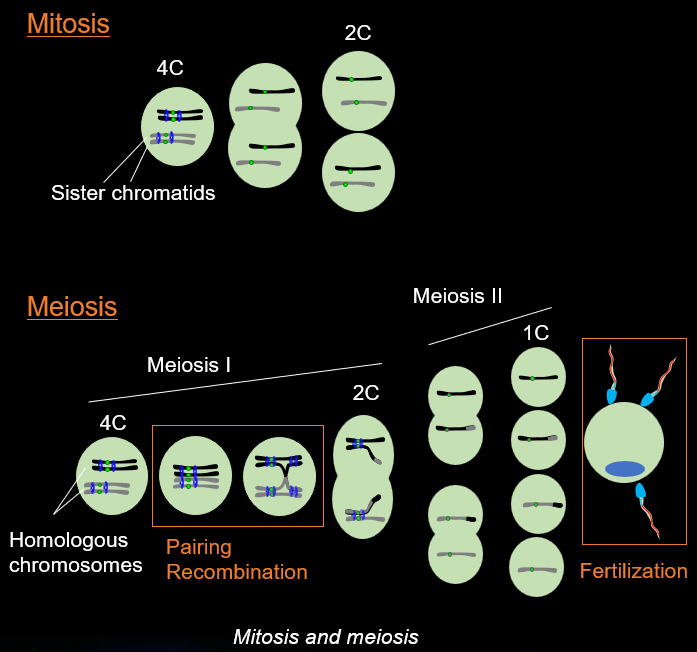
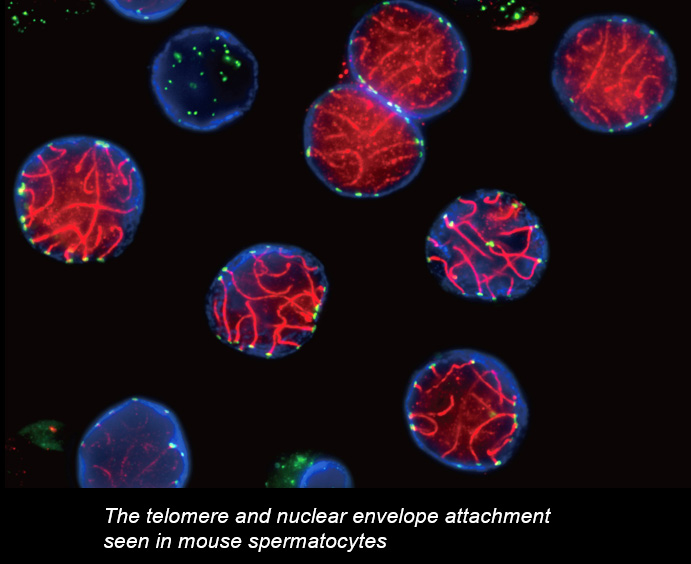
Chromosome movements in meiotic prophase I
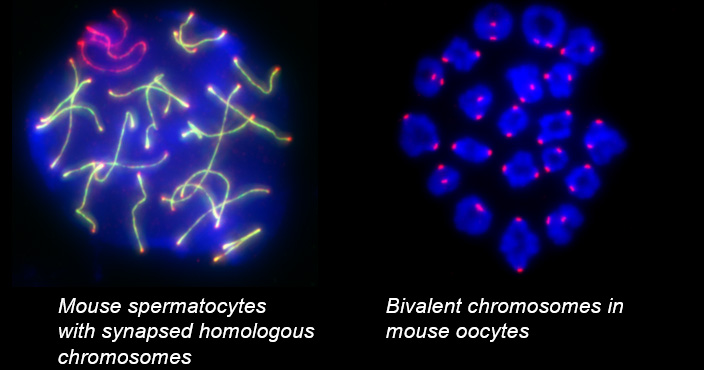
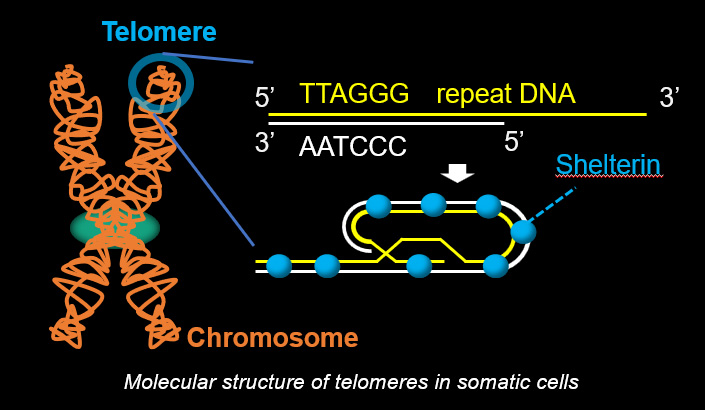
Telomeres, the terminal structures of chromosomes, are composed of repetitive DNA sequences and the sequence-specific DNA-binding protein complex Shelterin. Telomeres play important cellular roles, such as regulating DNA length, regulating cell lifespan, and suppressing the DNA-damage response. Recently, we found that a specialized protein complex called TERB1-TERB2-MAJIN specifically binds to telomeric DNA in meiotic prophase I. TERB1-TERB2-MAJIN physically links the telomeric DNA to the lipid bilayer that makes up the nuclear envelope. Telomeres on the nuclear envelope associate with cytoplasmic microtubule motor proteins through SUN1-KASH5, a transmembrane protein complex that spans the inner and outer nuclear envelopes. This transmembrane connection to the cytoplasmic motor proteins drives the chromosome movements in the nucleus, allowing homologous chromosomes to move around in order to find and pair with the correct partners.
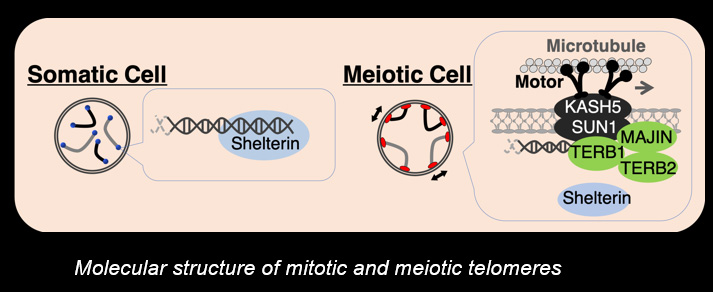
Homologous recombination
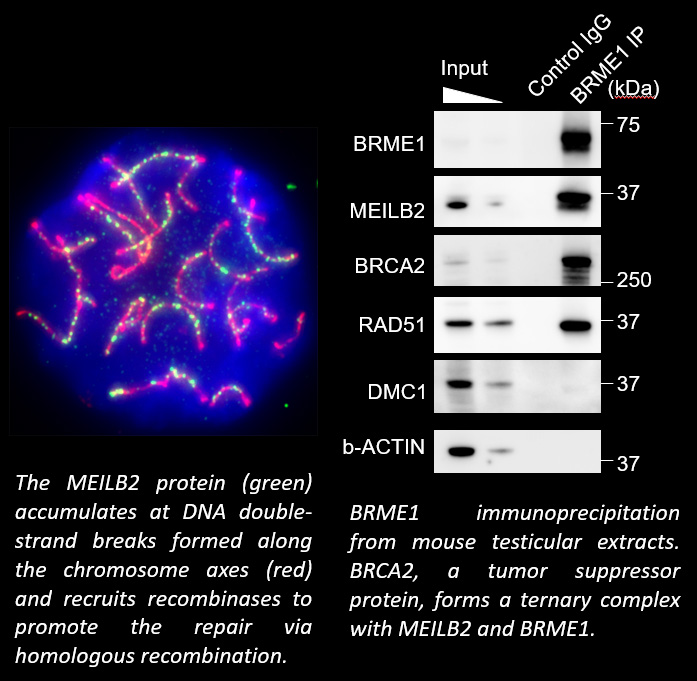
When pairs of homologous chromosomes are brought close together by telomere-driven chromosome movement, the DNA strands are exchanged and the homologous chromosomes acquire stronger physical association via homologous recombination. This physical bond, called a chiasmata, serves to physically hold the homologous chromosome pair together until the time when the homologous chromosomes are segregated during meiosis I. We found that BRCA2, a well-known tumor suppressor protein, forms a ternary complex with MEILB2 and BRME1, the meiosis-specific interactors of BRCA2, in order to facilitate homologous recombination by recruiting recombinases (RAD51 and DMC1).
Using Caenorhabditis elegans to investigate the molecular mechanisms of species survival
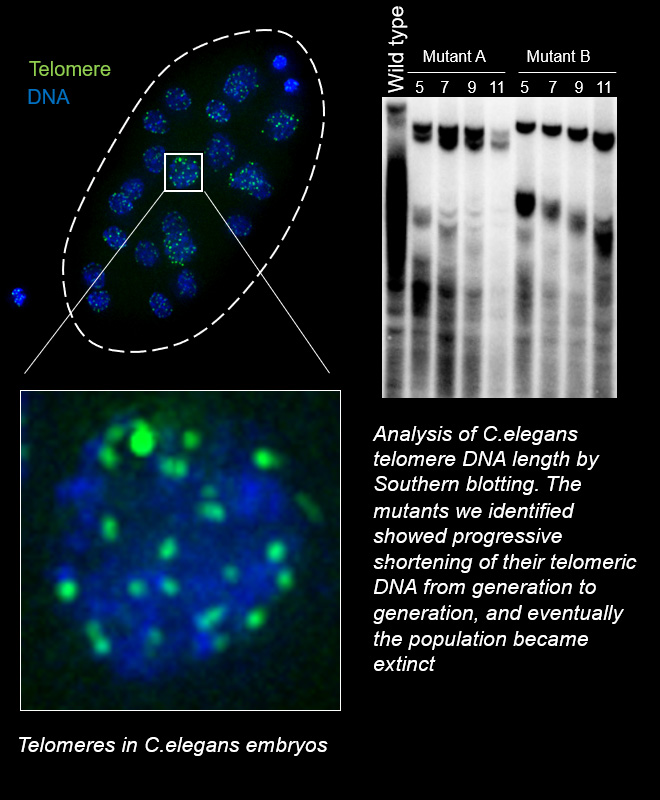
Telomeric DNA at the ends of chromosomes shortens with each cell division. When it reaches a specific limit, the cell stops dividing (Hayflick limit). Therefore, the length of telomeric DNA is thought to determine the lifespan of a cell. In special cells such as stem cells and cancer cells, which are capable of dividing semipermanently, the activation of telomerase or ALT (alternative lengthening of telomeres) ensures the lengthening of telomeres.
As I mentioned at the beginning, living things have a lifespan at the individual level, but as a species they can survive semi-permanently. Considering this at the telomere level, organisms must have a mechanism to extend telomere DNA across generations and to maintain telomere length at a constant level at the species level (telomere length homeostasis). The key regulations must lie in germ cells, the cells that pass DNA molecules on to the next generation.
To study telomere length regulation across generations, it is advisable to use model organisms with a short generation time. For example, in the case of mice, which are major mammalian model organisms in laboratories, the generation time is about 3 months. If we were to analyze a mutant whose telomere DNA is expected to become shorter each generation and to develop abnormalities after 10 generations, it would take 2.5 years to obtain the desired mice in the laboratory. If the hypothesis turns out to be wrong and the mouse obtained does not show any abnormalities, an enormous amount of time is wasted and your career as a scientist is over. For your security, in our laboratory, we use C. elegans, which has a short generation time of only 3 days, as a model organism for studying the transgenerational regulation of telomere length. C. elegans is a multicellular eukaryote just like human but has the advantage of being easy to genetically modify and being hermaphroditic, allowing it to grow in large numbers through self-fertilization.